PhD Top Stories
Debora Curci
Serum adalimumab levels after induction are associated with long-term remission in children with inflammatory bowel disease.
Doctoral Programme in Reproduction and Developmental Sciences
Inflammatory Bowel Disease (IBD), encompassing Crohn's disease (CD) and ulcerative colitis (UC) are chronic, relapsing disorders with progressive bowel damage leading to long-term disability. Children with IBD experience more severe disease than adults as they have more extensive disease, and more often need more intense pharmacological treatment with biologic agents. Among these, monoclonal antibodies targeting TNF-alpha play a pivotal role in the treatment of IBD, in particular, in patients with severe disease activity, unresponsive to standard therapies. Adalimumab, a fully humanized monoclonal antibody administered subcutaneously every two weeks, is effective in inducing and maintaining remission in children with IBD. Therapeutic drug monitoring (TDM), consisting in the measurement of drug concentration in patients blood, is an effective strategy to maximize the response rates and the association between serum adalimumab concentrations and the clinical response has already been demonstrated in adult patients with IBD but studies in children are lacking. In particular, it is not known if serum drug levels measured in the early phases of treatment can predict the long-term response in children.
In this context, this study aimed at assessing the predictivity of adalimumab concentrations measured at the end of induction and early during maintenance for long term response. Adalimumab concentrations in serum samples, collected both during the initial therapy (induction, week 4 of therapy) and during maintenance phases (week 22, 52 and 82), were measured using enzyme-linked immunosorbent assays (ELISA). Our results have shown that serum adalimumab levels at 4 weeks were associated with clinical remission during maintenance. In particular, higher serum adalimumab levels at week 4 were found for patients in clinical remission compared with patients with clinically active disease at week 22 (P = 0.0029), at week 52 (P = 0.003), and at week 82 (P = 0.003) of therapy (Figure 1).
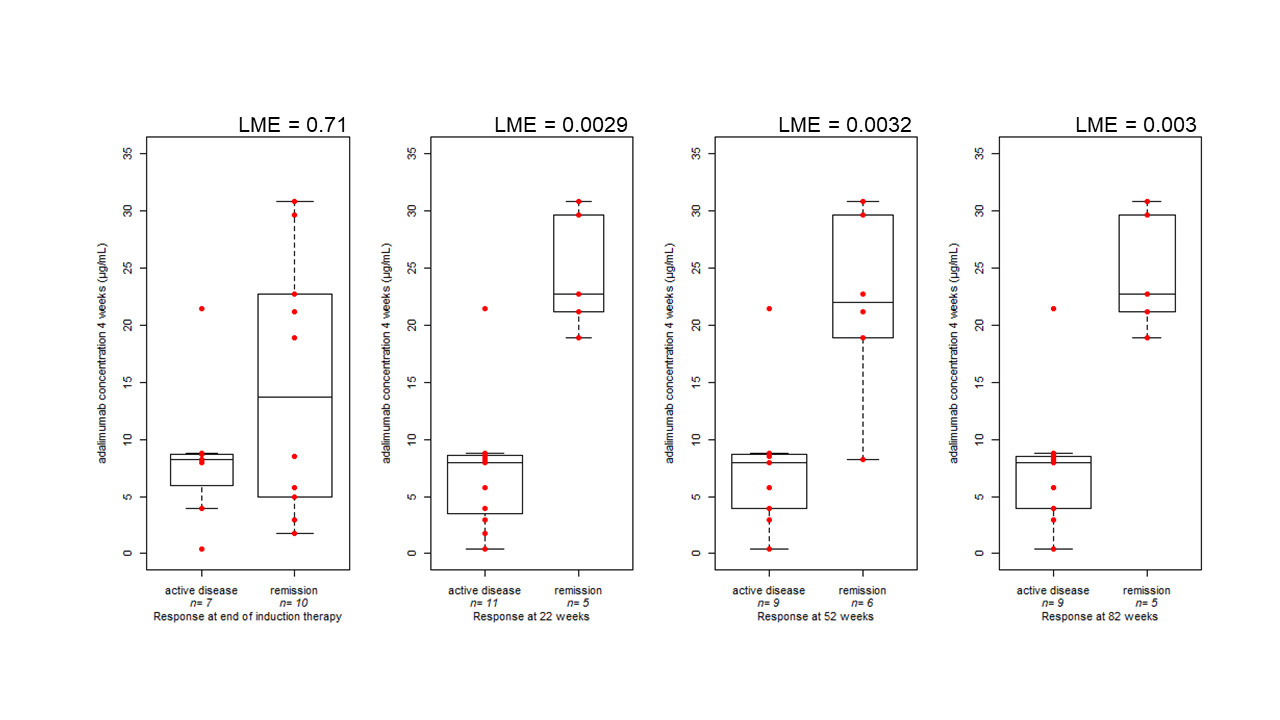
Figure 1: Boxplot comparing clinical disease activity at the end of induction (17 measurements in 13 patients), at 22 (16 measurements in 12 patients), 52 (15 measurements in 11 patients), and 82 weeks (14 measurements in 10 patients) and serum adalimumab concentration during induction therapy (4 weeks). The bold horizontal line represents the median value. Statistical significance was assessed by linear mixed effect model (LME), accounting for repeated measures
Similar results were also obtained considering adalimumab levels at week 22 and the clinical response at weeks 52 and 82 of therapy (P = 0.09; and P = 0.016, respectively) (Figure 2).
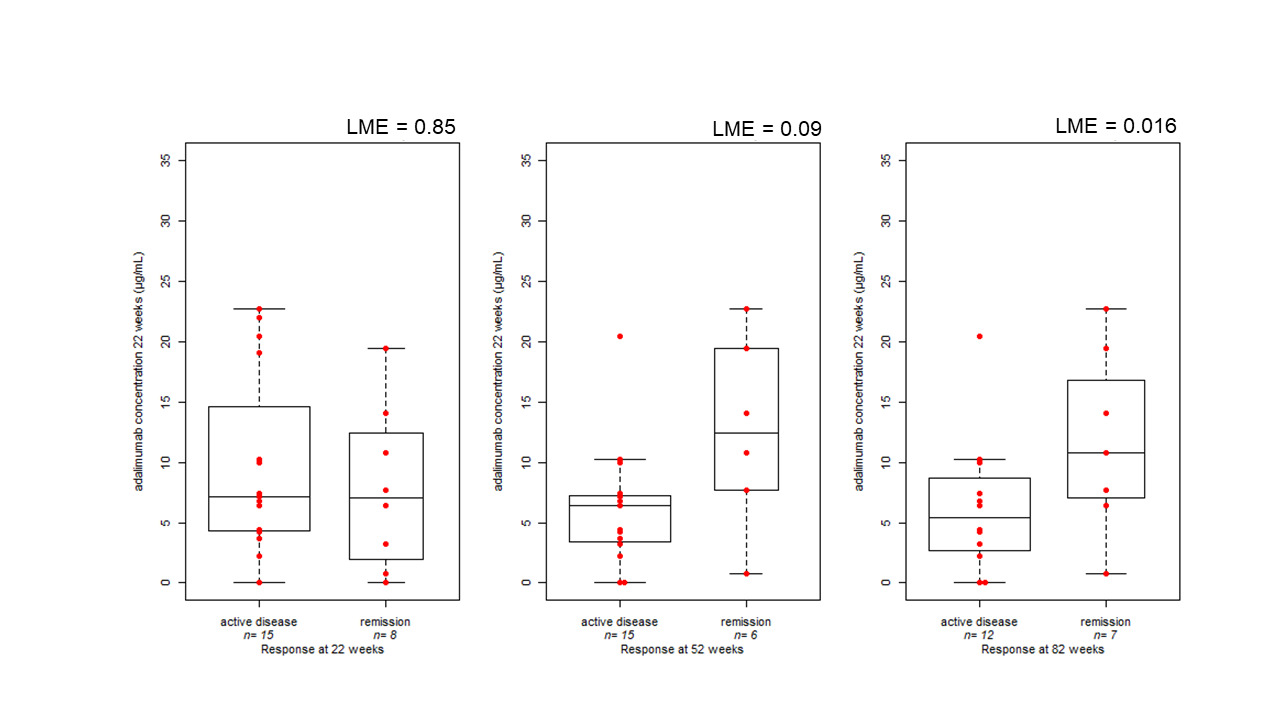
Figure 2: Boxplot comparing clinical disease activity at 22 (23 measurements in 19 patients), 52 (21 measurements in 18 patients), and 82 (19 measurements in 16 patients) weeks of treatment and serum adalimumab concentration during week 22. The bold horizontal line represents the median value. Statistical significance was assessed by linear mixed effect model (LME), accounting for repeated measures.
These results underlined the importance of TDM and in particular put in evidence that the determination of cutoff values for adalimumab concentration (above 13.85 and 7.54 µg/ml at week 4 and 22, respectively) emerged as a good predictor of long term remission (P< 0.05). Because anti-drug antibodies production can impact both on drug pharmacokinetics and pharmacodynamics by enhancing the drug clearance and could lead to a worse treatment response; in patients with adalimumab concentrations below 1.5 µg/mL, the presence of anti-drug antibodies was evaluated, showing a marginally significant inverse correlation with adalimumab levels (Spearman test, P = 0.073).
This work supports the utility of monitoring adalimumab concentrations at an early stage; adopting a proactive strategy for monitoring threapy may result in better long-term success rates with this expensive but effective therapy in children with IBD.
Authors and affiliations
Marianna Lucafň1, Debora Curci2, Matteo Bramuzzo3, Patrizia Alvisi4, Stefano Martelossi5, Tania Silvestri6, Veronica Guastalla7, Flavio Labriola4, Gabriele Stocco8, Giuliana Decorti1,72Reproductive and Developmental Sciences, University of Trieste, Trieste, Italy
3Gastroenterology, Digestive Endoscopy, and Nutrition Unit, Institute for Maternal and Child Health-Scientific Institute for Research, Hospitalization and Healthcare (IRCCS) “Burlo Garofolo”, Trieste, Italy
4Pediatric Gastroenterology Unit, Maggiore Hospital, Local health Centre (AUSL) Bologna, Bologna, Italy
5Pediatric Gastroenterology Unit, Cŕ Foncello Hospital, Treviso, Italy
6Single metropolitan laboratory (LUM) Autoimmunity and Allergy, Local Health Centre (AUSL) Bologna, Bologna, Italy
7Department of Medicine, Surgery, and Health Sciences, University of Trieste, Trieste, Italy
8Department of Life Sciences, University of Trieste, Trieste, Italy.
Contact
Debora Curci, email: debora.curci@phd.units.itReference
Marianna Lucafň, Debora Curci, Matteo Bramuzzo, Patrizia Alvisi, Stefano Martelossi, Tania Silvestri, Veronica Guastalla, Flavio Labriola, Gabriele Stocco and Giuliana Decorti"Serum Adalimumab Levels After Induction Are Associated With Long-Term Remission in Children With Inflammatory Bowel Disease."
Front. Pediatr. -, 04 May 2021
https://doi.org/10.3389/fped.2021.646671 10.3389/fped.2021.646671
Informazioni aggiornate al: 28.5.2021 alle ore 10:36
Contact: Webmaster - Università di Trieste pagina curata da: Research Doctorate

Piazzale Europa, 1 - 34127 - Trieste, Italia -
Tel. +39 040 558 7111 - P.IVA 00211830328
C.F. 80013890324 - P.E.C. ateneo@pec.units.it



