PhD Top Stories
Roman Vuerich
Promotion of schemic wound revascularization based on host-donor hybrid vessels
Doctoral Programme in Molecular biomedicine
Non-healing wounds are painful skin injuries that do not heal and often worsen over time. This is typically caused by the coexistence of underlying chronic conditions, such as diabetes and peripheral arteriopathies, which impede the wound's adequate vascularization. Without sufficient oxygen and nutrient supply provided by the blood, the wound cannot heal properly.
This is a common condition in people over 60 years old, at least as much as heart failure, with significant limitations in daily activities. Moreover, the incidence is increasing due to an increase in the average age of the population, cases of diabetes and chronic diseases. The economic burden of non-healing wounds is considerable, with approximately 3% of the global healthcare budget being spent on their treatment. In Italy alone, the cost of treating difficult wounds amounts to over 3 billion euros per year, as they often require specialized and costly therapies. Patients with difficult wounds may experience reduced work capacity and may require ongoing healthcare assistance.
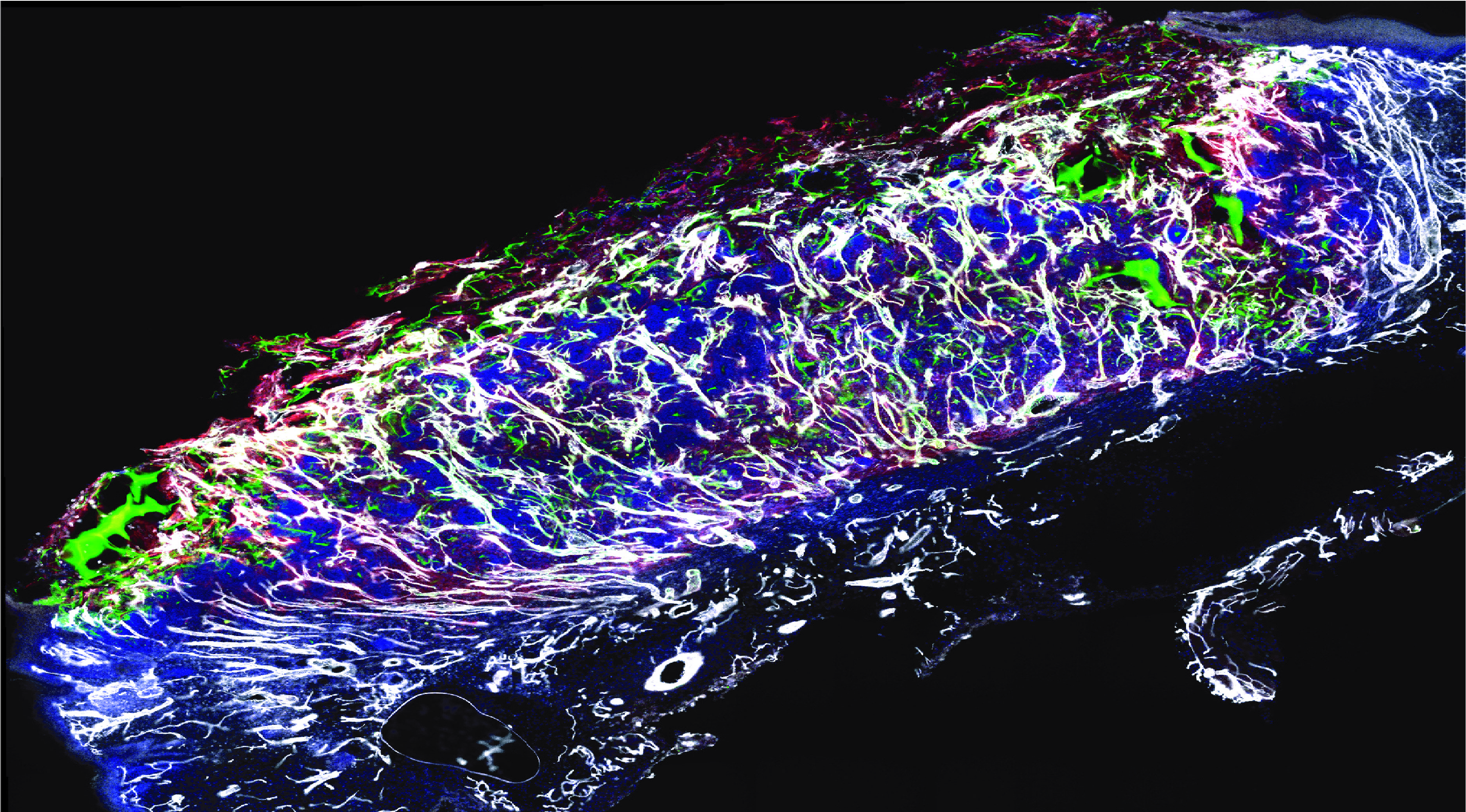
Figure 1Vascular network (in white) formed following wound treatment with adipose tissue cells (in green and red)
Current therapies available for non-healing wounds involve the use of skin substitutes to promote wound healing, but their effectiveness is hampered by the poor vascularization that typically underlies this condition. To address this issue and promote wound revascularization, we have developed a new therapy using Stromal Vascular Fraction (SVF). The SVF is a heterogeneous population of cells derived from fat that contains all the necessary building blocks for the formation of new blood vessels. In order to study the therapeutic effects of this new therapy, we developed a preclinical model of ischemic wound that closely simulates the pathology that occurs in humans. We then applied SVF cells taken from the patients' subcutaneous fat, obtained by liposuction, to the wound.
After just one week, the SVF cells were able to form a new, functional vascular network that was connected to the blood stream through pre-existing vessels in the wound (Figure 1). The restoration of normal blood supply following the formation of the new blood vessels significantly accelerated the healing process compared to conventional therapies, and allowed wound closure after only three weeks. Furthermore, we demonstrated that cells derived from diabetic patients, the primary target for this therapy, could be expanded in a bioreactor, preserving the therapeutic potential of SVF. Thus, we characterized the dual mechanism of action of our therapy. The cells that composed the vessels of SVF engrafted and expanded in the wound bed, directly contributing to new vessel formation. Additionally, a population of fibro-adipogenic progenitors secreted growth factors that further supported the expansion and function of the new vasculature. These data have important clinical implications, as they provide a steppingstone toward the reproducible and effective adoption of the SVF as a standard care for non-healing wounds.
Authors and affiliations
Roman Vuerich1/2, Elena Groppa1/#, Simone Vodret1, Nadja Annelies Ruth Ring1/*, Chiara Stocco3/4, Fleur Bossi5, Silvio Bicciato7, Chiara Agostinis5, Matteo Cauteruccio1,2, Andrea Colliva1, Mohammad Ramadan1, Francesca Simoncello6, Federica Benvenuti6, Anna Agnelli7, Franca Dore7, Flavia Mazzarol8, Massimo Moretti8, Alice Paulitti8, Silvia Palmisano3, NicolÚ De Manzini3, Mattia Chiesa9, Manuel Casaburo9, Angela Raucci9, Daniela Lorizio9, Giulio Pompilio9/10, Roberta Bulla2, Giovanni Papa3/4 Serena Zacchigna1,3,92Department of Life Sciences, PhD program in molecular biomedicine, University of Trieste, 34127 Trieste, Italy.
3Department of Medical, Surgical and Health Sciences, University of Trieste, 34127 Trieste, Italy.
4Plastic Reconstructive and Aesthetic Surgery Department, Ospedale di Cattinara, ASUGI, 34149 Trieste, Italy.
5Department of Biological Sciences (DBS), National University of Singapore (NUS), 14 Science Drive 4, 117543, Singapore.
6Cellular Immunology Laboratory, International Centre for Genetic Engineering and Biotechnology (ICGEB), 34149 Trieste, Italy.
7Nuclear Medicine Unit, University Hospital of Trieste - ASUGI, Trieste, Italy.
8VivaBioCell S.p.A., 33100 Udine, Italy.
9Centro Cardiologico Monzino Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), 20138 Milano, Italy.
10Department of Biomedical, Surgical and Dental Sciences, University of Milano, 20122 Milano, Italy.
#Current address: Scuola Internazionale Studi Superiori Avanzati (SISSA), 34136 Trieste, Italy.
*Current address: Ludwig Boltzmann Research Group SHoW Ė Senescence and Healing of Wounds, LBI Trauma, Vienna, Austria.
Contact
Roman Vuerich, email: ROMAN.VUERICH@phd.units.itReference
Vuerich R, Groppa E, Vodret S, Ring NAR, Stocco C, Bossi F, Agostinis C, Cauteruccio M, Colliva A, Ramadan M, Simoncello F, Benvenuti F, Agnelli A, Dore F, Mazzarol F, Moretti M, Paulitti A, Palmisano S, De Manzini N, Chiesa M, Casaburo M, Raucci A, Lorizio D, Pompilio G, Bulla R, Papa G, Zacchigna S.Ischemic wound revascularization by the stromal vascular fraction relies on host-donor hybrid vessels
NPJ Regen Med 10, 2023 Feb 11;8(1):8.
DOI: 10.1038/s41536-023-00283-6. PMID: 36774354; PMCID: PMC9922297.
Informazioni aggiornate al: 15.5.2023 alle ore 13:50
Contact: Webmaster - Università di Trieste pagina curata da: Research Doctorate

Piazzale Europa, 1 - 34127 - Trieste, Italia -
Tel. +39 040 558 7111 - P.IVA 00211830328
C.F. 80013890324 - P.E.C. ateneo@pec.units.it



