PhD Top Stories
Francesco Armillotta
Oxygen reduction and biomimesis: H-Bonding, Solvation, and the Elusive Hydroperoxyl Intermediate
Doctoral Programme in Physics
Catalysts are widely exploited for the synthesis of energy vectors and chemicals. Nevertheless, the underlying reaction mechanisms remain often beyond our insight limits. One example is the Oxygen Reduction Reaction (ORR), where oxygen can be reduced to either water or hydrogen peroxide via 2- or 4- electron pathways, yielding the formation of water or hydrogen peroxide:
O2 + 2H+ + 2e- → H2O2
O2 + 4H+ + 4e- → 2H2O
This process is at the basis of many biochemical and applicative catalytic mechanisms, and in particular the 4- electron pathway that yields water takes place also in the discharge process at the electrodes of some families of secondary (rechargeable) batteries, called metal-air batteries. During charge, instead, the reverse process occurs, namely the Oxygen Evolution Reaction (OER). Thus, the catalyst in the battery must be able to catalyze both reactions, and for this reason, needs to be bifunctional. Though, it is not clear yet what actually steers the reaction between the two competing pathways in ORR, including the role of a common intermediate, namely the hydroperoxyl (OOH). Aiming at investigating and establishing an easy way to tackle the reaction, the research group approached the problem using a single-atom catalyst based on cobalt-tetra-pyridyl-porphyrins self-assembled on graphene in ultra-high vacuum (UHV) conditions (Fig. 1A). Indeed, by using this metallorganic framework (MOF), there is the huge advantage of having the single cobalt atom as the only, well-defined active site, facilitating the interpretation of the observed features. The almost-free standing graphene support, grown by chemical vapor deposition at the (111) termination of an iridium single-crystal, guarantees the complete chemical inactivity, allowing the researchers to focus only on the MOF/GR system.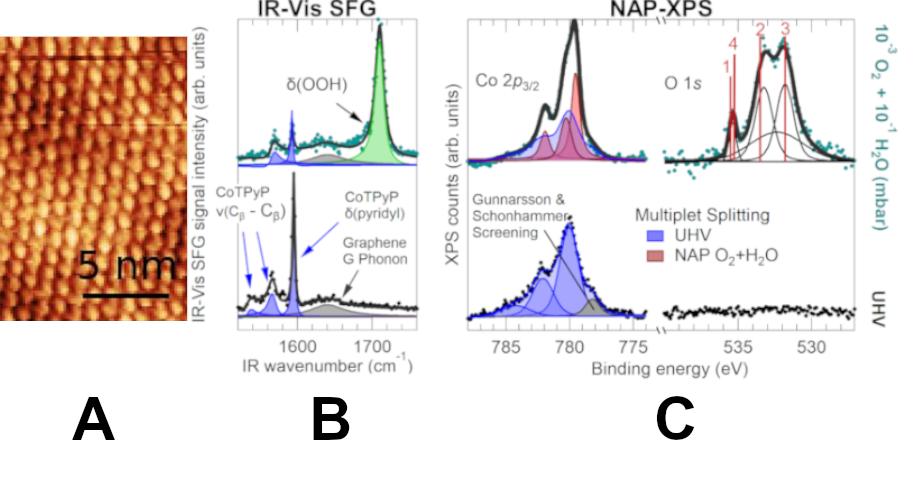
Figure 1: A) Scanning Tunneling Microscopy image of the CoTPyP/Gr/Ir(111) catalyst surface, where each “blob” is a CoTPyP molecule; B) IR-Vis SFG and C) NAP-XPS direct spectroscopic evidence of the formation of O2H at the Co sites.
FINDINGS The OOH ligand is stabilized at the Co sites via the formation of an OOH-H2O complex upon exposure of the surface to water (~0.1 mbar) and oxygen (~10-3 mbar) . The spectroscopic fingerprint (bending mode) of the hydroperoxyl-water complex @1712 cm-1 (Fig. 1B) was directly observed in sum-frequency generation (IR-Vis SFG) spectra, collected in situ. NAP-XPS measurements (Fig. 1C) performed at the HIPPIE beamline of the Max IV synchrotron radiation facility confirmed our thesis: the interpretation of the O 1s core level, corroborated by ab initio Density Functional Theory (DFT) calculations, is straightforward, and one-to-one attribution is provided to the many oxygen species (OOH-H2O, OOH-H2O, OOH-H2O, and H2O(g)). Complementary information is given by the analysis of the complex Co 2p core level lineshape that indicates bonding of the ligand at the cobalt site. Finally, a time-resolved IR-Vis SFG experiment revealed that the presence of water, stabilizing the complex through covalent and hydrogen bonding and dipole interactions, driving the energy transfer from the reaction site to the surrounding solvent with a time constant of the order of 10 ps. The work, just published in ACS Catalysis, was the fruit of an international collaboration involving the Lund University and the MAX IV synchrotron in Sweden, as well as the Politecnico di Milano. The project was coordinated by prof. Erik Vesselli with the contribution of prof. Maria Peressi and prof. Silvio Modesti from the Physics Department of University of Trieste. Remarkably, the first four authors of the paper (dr. Francesco Armillotta, dr. Davide Bidoggia, dr. Stefania Baronio and dr. Pietro Biasin) and the fifth one (dr. Antonio Annese) are Ph.D. students and a former Master student in Physics at our Department, respectively. The work was co-funded by Italian MUR (grant PRIN 2017KFY7XF), Universita` degli Studi di Trieste (grant FRA2021), Cineca, and the Swedish Research council and Governmental Agency for Innovation Systems.
Authors and affiliations
Francesco Armillotta1, Davide Bidoggia1, Pietro Biasin1, Antonio Annese1, Mattia Scardamaglia2, Suyun Zhu2, Benedetto Bozzini3, Silvio Modesti1, Maria Peressi1, Erik Vesselli1,4,52MAX IV Laboratory, Fotongatan 8 - 224 84 - Lund (Sweden).
3Department of Energy, Politecnico di Milano, 20156 Milano (Italy).
44CNR-IOM, Area Science Park, S.S. 14 km 163.5, 34149 Basovizza, Trieste (Italy).
5Center for Energy, Environment and Transport Giacomo Ciamician - University of Trieste (Italy).
Contact
Francesco Armillotta, email:francesco.armillotta@phd.units.itReference
Francesco Armillotta, Davide Bidoggia, Stefania Baronio, Pietro Biasin, Antonio Annese, Mattia Scardamaglia, Suyun Zhu, Benedetto Bozzini, Silvio Modesti, Maria Peressi, Erik VesselliSingle Metal Atom Catalysts and ORR: H-Bonding, Solvation, and the Elusive Hydroperoxyl Intermediate
ACS Catalysis 12,13, 7950–7959 (2022)
DOI: 10.1021/acscatal.2c02029
Informazioni aggiornate al: 14.4.2023 alle ore 14:06
Contact: Webmaster - Università di Trieste pagina curata da: Research Doctorate

Piazzale Europa, 1 - 34127 - Trieste, Italia -
Tel. +39 040 558 7111 - P.IVA 00211830328
C.F. 80013890324 - P.E.C. ateneo@pec.units.it



